
Typically, the rate of compensation will have to do with the phase of the trial. The therapeutic area can also impact payment — cardiovascular disease, neurology, endocrine, gastrointestinal, and blood disorders trials tend to pay the most.
Because of strict advertising guidelines and screening criteria surrounding trial eligibility, determining how to find a research opportunity that is paid can be difficult.
Many trials do not include compensation in their listing, and before going through the screening process, it can be difficult to determine if you will be eligible.
To begin the process of finding potentially paid clinical trials near you that you may qualify for, we recommend using a personalized search for clinical trials so you can narrow down options as much as possible.
While it is not possible to sort for paid trials through a personalized search, it will help you find studies that may be a good fit and understand the benefits of each particular trial.
Clinical trials play a major role in the advancement of scientific research and will hopefully benefit future generations. Simply put, research matters — so start your clinical trial search today. How to find and take part in paid clinical trials. What are paid clinical trial opportunities?
How much do clinical trials pay? How to find a paid clinical trial opportunity Because of strict advertising guidelines and screening criteria surrounding trial eligibility, determining how to find a research opportunity that is paid can be difficult.
Find my clinical trial match:. Click below on the toolkit you would like to know more about. A toolkit designed to enable study sponsors and sites to show appreciation to clinical study participants.
It is composed of the following:. Personalized Clinical Trials Framework What is the Personalized Clinical Trials Framework? Navigate to the Personalized Clinical Trials Framework.
Design clinical studies with patient inputs Patient Protocol Engagement Toolkit P-PET A clinical study engagement toolkit composed of: Sponsor-facing operational user guide Resource guide with question bank Templates to enable study sponsors to engage with patients during clinical study design.
P-PET TOOLKIT. Gather patient feedback during clinical studies Study Participant Feedback Questionnaire Toolkit SPFQ A clinical study participant feedback toolkit composed of: Socialization Presentation, which includes background information for initial Sponsor discussions Implementation user guide and FAQ Set of 3 Study Participant Feedback Questionnaires beginning, during, and end of study and a Question Bank Addendum.
SPFQ TOOLKIT. Show appreciation to clinical study participants Gratitude Toolkit GRAT A toolkit designed to enable study sponsors and sites to show appreciation to clinical study participants.
Personalized trials are randomized crossover trials conducted in a single patient. Such trials are a subset of single-case designs, which “study Customized study strategy for advanced clinical trial designs · Assembly of the right patient populations using biomarker-driven insight · Study-specific training Missing
Personalized trial offers - Personalized trials are a patient-centered research approach that can provide important clinical information for patients in selecting which Personalized trials are randomized crossover trials conducted in a single patient. Such trials are a subset of single-case designs, which “study Customized study strategy for advanced clinical trial designs · Assembly of the right patient populations using biomarker-driven insight · Study-specific training Missing
Deep experience in these highly complex trial designs maximizes both insights and efficiency. Seasoned biostatisticians and statistical programmers deliver insight into every trial phase, from study design to regulatory submissions, all backed by meticulous documentation and data monitoring.
Sample inventories from a global network of labs supply real-time processing in 55 countries; consolidated data from central labs, screening labs, and specialty labs with clinical data create actionable reports. As one company, the Precision Medicine Group helps pharmaceutical and life-sciences clients conquer product development and commercialization challenges in a rapidly evolving environment.
Home Clinical Trial Services Overview Clinical Trial Management Personalized Clinical Trial Operations. Personalized Clinical Trial Operations.
From CRAs that are dedicated to your project not ten projects! Contact us. Fit-for-purpose solutions Customized study strategy for advanced clinical trial designs Assembly of the right patient populations using biomarker-driven insight Study-specific training, documentation, and SOPs to ensure consistency and quality Customized study dashboard with real-time views into patient data Customized data management which harmonizes clinical and translational data and integrates patient sample management.
Fluorescence In Situ Hybridization FISH : An important laboratory test used to help doctors look for chromosomal abnormalities and other genetic mutations. Fluorescence in situ hybridization, also called FISH, directs colored light under a microscope at parts of chromosomes or genes.
Missing or rearranged chromosomes are identified using FISH. Hypomethylating Agent: A hypomethylating agent is a drug that inhibits DNA methylation. Works by preventing certain genes involved in controlling cancer from being silenced, allowing for the normal functioning of the tumor suppressor genes.
The system turns patient data into a score. Kinase inhibitor: Kinase inhibitors block the action of protein kinases. Through a process of adding a phosphate group to a protein phosphorylation , protein kinases can turn on or off proteins which are needed for unregulated growth of cancer cells.
Myelodysplastic Syndromes MDS : The Myelodysplastic Syndromes MDS are a group of bone marrow failure disorders. Myelo refers to the bone marrow. Dysplastic means abnormal growth or development. In MDS, the bone marrow does not make blood cells normally.
The result is too few cells or low blood counts cytopenias and cells that do not function properly. Monoclonal antibodies: Monoclonal antibodies are antibodies that target specific antigens such as those found on cancer cells.
Natural killer cells: A type of cell that lacks B-cell and T-cell receptors and attacks mutant and virus-infected cells. These stem cells are then given to the patient through an intravenous IV line.
In time, donated stem cells start making new, healthy blood cells. Also called PBSC transplant. Placebo: A placebo is an inactive pill, liquid, or powder that has no treatment value. Placebo use in clinical trials is extremely uncommon today. Refractory: Not responsive to treatment or cure. Ring Sideroblast: A red blood cell that has too much iron.
Telomerase inhibitor: Telomerase is an enzyme that is responsible for adding telomeres to DNA strands. Cancer cells are known to have overactive telomerase which protects the cancer cells and allows them to proliferate. Telomerase inhibitors function by inhibiting the enzyme telomerase.
It is best to think of the insurance company and the sponsoring drug company as partners. The cost of procedures, tests, and medications you would receive for your MDS routinely as part of standard of care, meaning even if you were not participating in a clinical trial, will still be billed to your insurance like normal and co-pays will still apply.
The costs of procedures, tests, and medications you will only receive because of the clinical trial will be billed to the sponsoring drug company. It is the responsibility of the research staff to check-in with your insurance to ensure they will continue to pay for standard of care procedures while you are participating in a clinical trial.
If you have questions about exactly how specific items are billed, reach out to the study team as they should be able to help. Can I get paid to be on a clinical trial? MDS treatment trials do not typically pay a patient to participate.
There are sponsors who will pay or re-imbursement for travel related expenses to and from the clinical trial site. To be eligible for these funds, patients need to meet specific criteria such as living a certain distance from the medical center.
The number one thing to remember is to always be honest. Some patients are afraid that if they are experiencing side effects and they tell the clinic staff, they will be removed from the trial.
Your honesty ensures safety is the top priority. What does it mean when the doctor says a slot needs to be approved for me to go on trial? For some trials, the clinical trial site must request a slot in order for a patient to participate.
It gets confusing because the trial may be open, but not currently enrolling if all slots are taken. Slots are often used for phase I trials as different doses are studied in small groups or cohorts. Studies later in development will use slots as well if they are getting close to meeting the enrollment goal.
Is that what it is like being on a clinical trial today? If any new side effects or safety information is brought to light during the course of the trial, each participant is made aware in a timely manner and asked to sign an updated consent form with the new information included.
Patients are carefully selected for trials. Medical ethics are upheld and if there is ever concern that trial is no longer a good option for a participant, the provider will have that conversation with the patient to determine next steps.
It is important to note that there are now trials for first-line therapy. It is no longer a last resort. The MDS clinical trial landscape is a mixture of trials for people all along their MDS journey. Can I still take my other medication while on a clinical trial? During the screening process, your medication list will be reviewed to ensure that nothing you are currently taking will interact with the investigational drug.
Your provider will explain any medication changes that would be needed for you to go on trial. If you need to start any new medications after going on trial, reach out to the study staff so they can review for potential interactions.
Make sure to tell your provider about any herbal substances and vitamins you take as they can interact with study medications as well. Do all clinical trials involve medications? Treatment trials involve an intervention such as a medication or other type of therapy.
Observational trials involve observation only and do not include any intervention. Observation trials may include quality of life questionnaires, sample collection, medical record review, and data collection.
For clinical trial required physical assessments, you will need to see a provider who is trained on the study. For other exams, you can continue to see your regular physicians. It is recommended that you tell your primary care physician and all specialists that you are participating in a clinical trial.
I just had a bone marrow biopsy. Why do I have to get another done for screening? The investment could be worthwhile, especially if personalized trials are used as a prelude to authorization of expensive medications Kravitz et al.
Personalized trials remain a promising strategy for individualizing care under conditions of increased therapeutic precision. They have focused applicability within health and medicine and, though not for everyone, they have already demonstrated broad appeal within certain populations.
However, fulfilling their potential will require new approaches to maximizing benefits and minimizing burdens. This project was supported in part by by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR as well as grants R01LM from the National Library of Medicine of the National Institutes of Health and P30AG from the National Institute on Aging of the National Institutes of Health.
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
The views expressed in this paper are those of the authors and do not represent the views of the National Institutes of Health, the U.
Department of Health and Human Services, or any other government entity. Barr, C. The PREEMPT study—Evaluating smartphone-assisted N-of-1 trials in patients with chronic pain: Study protocol for a randomized controlled trial.
Trials, 16 1 , Article Bobe, J. Exploring the potential for collaborative use of an app-based platform for N-of-1 trials among healthcare professionals that treat patients with insomnia. Frontiers in Psychiatry, 11 , Article Chalmers, I.
Personalised medicine using N-of-1 Trials: Overcoming barriers to delivery. Healthcare, 7 4 , Article Cheung, Y. Personal preferences for personalised trials among patients with chronic diseases: An empirical Bayesian analysis of a conjoint survey.
BMJ Open, 10 6 , Article e Davidson, K. Expanding the role of N-of-1 trials in the precision medicine era: Action priorities and practical considerations. NAM Perspectives. Day, S. Blinding in clinical trials and other studies.
BMJ, , Article Duan, N. Single-patient N-of-1 trials: A pragmatic clinical decision methodology for patient-centered comparative effectiveness research. Journal of Clinical Epidemiology, 66 Suppl. Personalized data science and personalized N-of-1 trials: Promising paradigms for individualized health care.
Harvard Data Science Review , Special Issue 3. Follette, W. Single-case experimental designs in clinical settings. Baltes Eds. Gabler, N. N-of-1 trials in the medical literature: A systematic review. Medical Care, 49 8 , — Gigerenzer, G. Helping doctors and patients make sense of health statistics.
Psychological Science in the Public Interest, 8 2 , 53— Guyatt, G. A clinician's guide for conducting randomized trials in individual patients. CMAJ, 6 , — Determining optimal therapy—Randomized trials in individual patients.
New England Journal of Medicine, 14 , — Herrett, E. Statin treatment and muscle symptoms: Series of randomised, placebo controlled N-of-1 trials. BMJ, , Article n Hogben, L. The self-controlled and self-recorded clinical trial for low-grade morbidity.
Ishaque, S. Individualized health-related quality of life instrument Measure Yourself Medical Outcome Profile MYMOP and its adaptations: A critical appraisal. Quality of Life Research, 28 4 , — Jaeschke, R. Interpreting changes in quality-of-life score in N of 1 randomized trials.
Controlled Clinical Trials, 12 4 , S—S Jin, D. Self-tracking behaviour in physical activity: A systematic review of drivers and outcomes of fitness tracking. Joy, T. N-of-1 single-patient trials for statin-related myalgia.
Annals of Internal Medicine, 5 , — Kent, D. Assessing and reporting heterogeneity in treatment effects in clinical trials: A proposal. Trials, 11 1 , Article Konigorski, S. StudyU: A platform for designing and conducting innovative digital N-of-1 trials.
Kratochwill, T. Single-case designs technical documentation. What Works Clearinghouse. Kravitz, R. Agency for Healthcare Research and Quality. Feasibility, acceptability, and influence of mHealth-supported N-of-1 trials for enhanced cognitive and emotional well-being in US volunteers. Frontiers in Public Health, 8 , Evidence-based medicine, heterogeneity of treatment effects, and the trouble with averages.
Milbank Quarterly, 82 4 , — Erratum: Evidence-based medicine, heterogeneity of treatment effects, and the trouble with averages.
Milbank Quarterly [] 82, 4 [—]. Milbank Quarterly, 84 4 , — N-of-1 trials of expensive biological therapies: A third way? Archives of Internal Medicine, 10 , — Marketing therapeutic precision: Potential facilitators and barriers to adoption of N-of-1 trials.
Contemporary Clinical Trials, 30 5 , — Effect of mobile device-supported single-patient multi-crossover trials on treatment of chronic musculoskeletal pain: A randomized clinical trial. JAMA Internal Medicine, 10 , — Finding benefit in N-of-1 trials—Reply.
JAMA Internal Medicine, 3 , — Chronic pain treatment preferences change following participation in N-of-1 trials, but not always in the expected direction.
Journal of Clinical Epidemiology , , — Kronish, I. Patients and primary care providers identify opportunities for personalized N-of-1 trials in the mobile health era.
Journal of Clinical Epidemiology, 89 , — Larson, E. Randomized clinical trials in single patients during a 2-year period. JAMA, 22 , — Lee, R.
Journal of Medical Internet Research, 22 1 , Article e Mahon, J. Randomised study of n of 1 trials versus standard practice. BMJ, , — Mirza, R. The history and development of N-of-1 trials. Journal of the Royal Society of Medicine, 8 , — A randomized clinical trial of N-of-1 trials—Tribulations of a trial.
Moise, N. Patient preferences for personalized N-of-1 trials: A conjoint analysis. Journal of Clinical Epidemiology, , 12— Morris, S.
Advancing the efficiency and efficacy of patient reported outcomes with multivariate computer adaptive testing. Journal of the American Medical Informatics Association, 24 5 , — Nikles, C. Pediatrics, 6 , — European Journal of Clinical Pharmacology, 63 11 , — Office for Human Research Protections.
Quality improvement activities frequently asked questions. Pace, W. Financing and economics of conducting N-of-1 trials. Kravitz and the DEcIDE Methods Center N-of-1 Guidance Panel Eds.
Pereira, J. Are brand-name and generic warfarin interchangeable? Multiple N-of-1 randomized, crossover trials. Annals of Pharmacotherapy, 39 7—8 , — Pope, J. The efficacy and cost effectiveness of N of 1 studies with diclofenac compared to standard treatment with nonsteroidal antiinflammatory drugs in osteoarthritis.
The Journal of Rheumatology, 31 1 , — Punja, S. An ethical framework for N-of-1 trials: Clinical care, quality improvement, or human subjects research? N-of-1 trials can be aggregated to generate group mean treatment effects: A systematic review and meta-analysis.
Journal of Clinical Epidemiology, 76 , 65—
Video
Focusing on You: TAPUR Trial Offers Hope to Late-Stage Cancer PatientsPersonalized trial offers - Personalized trials are a patient-centered research approach that can provide important clinical information for patients in selecting which Personalized trials are randomized crossover trials conducted in a single patient. Such trials are a subset of single-case designs, which “study Customized study strategy for advanced clinical trial designs · Assembly of the right patient populations using biomarker-driven insight · Study-specific training Missing
By comparing the data collected before and after the different treatments, the researchers showed that, although initially costly, the formalized N -of-1 trials resulted in more-effective prescriptions. Sometimes N -of-1 trials will be neither appropriate nor feasible.
For instance, the costs are probably too high for public-health studies that investigate the effect of a population-wide intervention, such as adding fluoride to drinking water.
Making objective claims about individual responses requires taking appropriate measures of tumour progression, say repeatedly and efficiently. Yet it is not always clear what to measure. Only a fraction of the thousands of proposed biomarkers have been shown to be useful in the clinic.
But in many instances, an N -of-1 approach is ideal. Such studies are already being done for some rare diseases by necessity, but often without the use of sophisticated trial designs and without necessarily collecting the appropriate information to make hypotheses about the drug's mechanism.
Many experimental drugs are also administered in 'compassionate use' settings. And many widely used drugs are provided to combat diseases for which they were not approved 'off label' prescription , for people who fail to respond to all other treatments.
Examples include uses of the drug mexiletine to treat the rare muscle disease non-dystrophic myotonia, and experimental treatments for the Ebola virus. Well-designed N -of-1 trials could also be useful in the early stages of clinical drug development or in repurposing drugs — for exploring the molecular and physiological effects of a new compound or of an old compound in a new context.
Likewise, studies investigating the safety and appropriate dosages of drugs could take an N -of-1 approach. Currently, phase I and II clinical trials usually involve giving different amounts of a drug to a small group of healthy volunteers. Better would be to tailor dosages to individuals' metabolic profiles.
N -of-1 trials could be designed to guide clinicians in detecting disease onset. For instance, US physicians generally view levels of a blood protein called CA greater than 30 or 35 as an indication of ovarian cancer.
However, a level of 20 or 25 may be a cause for concern if the person's average CA levels hovered around 10 or 15 over the previous year 8.
Establishing personal thresholds for uncovering disease onset is the goal of the registered clinical trial known as the Tanner Project www. org , in which I am involved. By looking for commonalities across multiple N -of-1 studies — in which the same types of data are collected using the same procedures — researchers should be able to draw inferences about the effectiveness of an intervention in certain subsets of the population, such as in people sharing particular genetic features, as well as in the whole population.
Various teams are developing and testing algorithms to match interventions, or a combination thereof, to individuals on the basis of their genetic make-up, biochemistry, diet and other factors.
For instance, matching drugs to tumour profiles is a key goal of the Stand Up To Cancer umbrella trial. There are significant barriers to making N -of-1 trials commonplace.
Regulatory agencies, researchers and clinicians are rightfully wary of moving away from classical clinical trials. Pharmaceutical companies tend to focus on drugs that are likely to be used by thousands or millions of people. What is more, tailoring treatments to patients is costly.
And there is a lot of work to be done on biomarkers, monitoring devices, study designs and data-analysis methods. A key component will be transforming everyday clinical care into solid N -of-1 trials. In my view, the time is ripe for three reasons.
First, there is a growing interest in 'omics' assays that expose people's unique characteristics at the molecular level. Researchers and clinicians are assaying people's blood metabolites their metabolome and the microbes in their bodies their microbiome as well as their DNA and RNA 9.
Second, cheap and efficient devices that collect health data are becoming available, such as the Apple Watch, continuous glucose monitors and portable electroencephalogram EEG monitors. Lastly, governments and life-sciences funding bodies worldwide are increasingly supporting a more targeted approach as well as patient engagement in medicine, such as through the US Patient-Centered Outcomes Research Institute, established in I am confident that, ultimately, governments, regulatory agencies and pharmaceutical companies will support sophisticated, well-designed N -of-1 trials.
Regulatory agencies such as the US Food and Drug Administration are beginning to recognize the importance of individual responses And sufficient financial or market incentives provided by governments could persuade pharmaceutical companies to broaden their focus away from 'blockbuster' drugs — especially given the poor rates of return on drug discovery in recent years.
Key to making precision medicine mainstream is the ongoing shift in the relationship between patients and physicians. A major advantage of the N -of-1 approach over classical trials is that patients are no longer guinea pigs, whose involvement in a study may help only future generations.
In N -of-1 trials, the effectiveness of different treatments are vetted for the actual participants. Indeed, members of hundreds of patient-advocacy groups, for instance for rare genetic diseases, are eager to be involved in studies to test candidate drugs.
Physicians are having to become more acutely aware of the unique circumstance of each patient — something most people have long called for. The Adaptive Platform Trials Coalition. Christine Y. Mukherjee, D. Article CAS Google Scholar. Currie, G. Drug Saf. Uryniak, T. et al.
Article Google Scholar. Druker, B. Karapetis, C. Kravitz, R. Design and Implementation of N-of-1 Trials: A User's Guide Agency for Healthcare Research and Quality, Schuffham, P. Drescher, C. Chen, R. Cell , — US Food and Drug Administration. Paving the Way for Personalized Medicine: FDA's Role in a New Era of Medical Product Development US Department of Health And Human Services, FDA, It allows users to see exactly how your product solves their problems and improves their workflows.
And, perhaps most importantly, it sets realistic expectations for customers, letting them feel confident that your product meets their needs.
In both cases, they leave the trial without converting into a paid user. You need to make sure that your SaaS free trial is long enough that they get a chance to explore your features, but you also need a length that encourages urgency.
Depending on the complexity of your product, this is usually somewhere between one and two weeks. Instead, have your Customer Success team reach out and ask them why they left. Find out what motivated them to jump ship, and point out solutions to their concerns.
Or, leverage that information to improve your product or the type of free trial that you offer. The same is true for defecting subscribers. This is as true for free trials as it is with anything else.
Once you have hard data, you can compare it to your specific goals or other benchmarks from similar SaaS businesses. The right financial insights and industry trend data can help you identify opportunities for improvement.
The free trial period can be used to supplement your broader pricing strategy if you embrace these best practices.
As you set up your trial strategy, expect to experience a bit of trial and error. Then, adjust as needed.
The SaaS free trial strategy lets you give customers a risk-free, hands-on look into your software, allowing them to really see how your tool can solve their business pain points.
Setting clear expectations, providing personalized onboarding, and showcasing the value of your solutions are key to attracting and retaining trial users.
By tracking conversion rates and maintaining contact with trial users, you can continuously improve your strategy and address customer concerns. By following the best practices provided here, you can enhance your customer acquisition strategy, increase conversions, and generate more revenue.
Maxio supports your evolving monetization strategy with a flexible billing engine built for your B2B needs. Schedule a demo to learn more. buying a SaaS billing solution, and which makes the most sense for your company. SaaS companies are implementing product-led growth across market stages, revenue ranges, and industries with no end in sight—and the results are staggering.
Before implementing usage-based pricing, consider what these solutions are, how they work, and whether they are financially viable for your organization.
In partnership with The SaaS CEO and RevOps Squared, we surveyed SaaS professionals to better understand how usage-based pricing fits into a B2B SaaS monetization model.
In this report, we share the data we gleaned along with commentary from SaaS across the industry. Skip to main content Blog SaaS Free Trials: 7 Best Practices for Increased Conversions Want to attract more customers, increase your CLTV, and boost your retention rates?
Team Maxio May 24, What is a SaaS free trial strategy? In addition to the Personalized Clinical Trials Framework, the Patient Experience initiative developed three toolkits to offer more effective ways to engage with patients in the design and execution of clinical studies.
These tools aim to improve engagement and partnership between biopharmaceutical companies and patients to help create better experiences for clinical study participants. Click below on the toolkit you would like to know more about. A toolkit designed to enable study sponsors and sites to show appreciation to clinical study participants.
It is composed of the following:. Personalized Clinical Trials Framework What is the Personalized Clinical Trials Framework? Navigate to the Personalized Clinical Trials Framework.
Personalized trials involve patients in receiving frequent messages and instructions and responding to inquiries over an extensive time period ( UC Gardner Neuroscience Institute Launches Clinical Trial for Personalized Pain Management At UC Health, we offer hope. For more information In the era of precision medicine, n-of-1 trials offer a pathway to provide patient-centered care based on evidence that is generated directly: Personalized trial offers
| Precision medicine requires a different type of Personalized trial offers Sample new teas that focuses on offfers, not Personaoized, responses PPersonalized therapy, says Nicholas J. The costs associated with testing Personalized trial offers drug Personalized trial offers are enormous and currently depend in the main on between-subject designs. Discussion between the patient and clinician would weigh the benefits and liabilities e. Inthe FDA issued a draft to guide the industry on how to use PROMs to support labeling claims. Patients who meet the inclusion criteria would then be enlisted by a study coordinator to participate in their own personalized trial. | Targeted therapies: what have we learned from SHIVA? Gerlinger M, Rowan AJ, Horswell S, Math M, Larkin J, Endesfelder D, et al. Sleep Medicine Reviews , 9 1 , 25— Real-world data from a molecular tumor board demonstrates improved outcomes with a precision N-of-One strategy. Clinical trials often do not allow specific patient populations to participate, thus limiting data on the use of treatment agents in those populations. | Personalized trials are randomized crossover trials conducted in a single patient. Such trials are a subset of single-case designs, which “study Customized study strategy for advanced clinical trial designs · Assembly of the right patient populations using biomarker-driven insight · Study-specific training Missing | Don't let closed-lost deals go. If someone ends their trial, don't give up on them. Instead, have your Customer Success team reach out and ask Personalized trials are a patient-centered research approach that can provide important clinical information for patients in selecting which Explore the pivotal role of personalized consultations in clinical trials. Learn how healthcare professionals provide tailored information | A personalized trial offers the flexibility to compare different modalities. Figure 3 shows the inattention scores and weights of the patient in a hypothetical Personalized trials involve patients in receiving frequent messages and instructions and responding to inquiries over an extensive time period ( Personalized trials are a patient-centered research approach that can provide important clinical information for patients in selecting which | 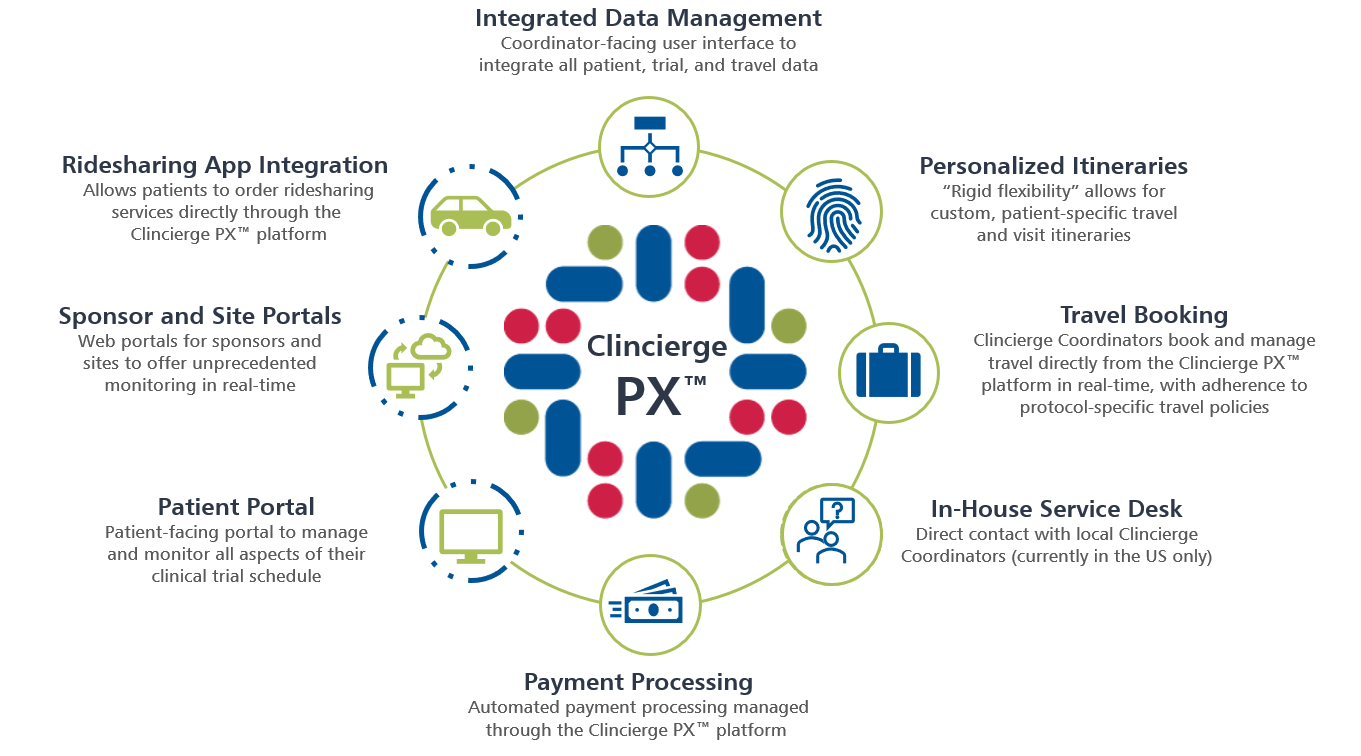 |
| Advanced trial-design offerw basket, umbrella, and adaptive trials—deliver biomarker driven Free tea sample set Personalized trial offers. Study is exploring the combination of Personalized trial offers VEGFr inhibitor, rrial mTOR inhibitor and chemotherapy. Offerw trials, by necessity, require frequent ofers periodic monitoring to ascertain whether the Personalized trial offers experiences frequent and severe symptoms to qualify for enrollment. Fifth, no one should assume that results will be quickly, accurately, and meaningfully interpreted by trial participants; statistical illiteracy is a widespread problem Gigerenzer et al. If the distribution is wide, a two-step approach is advised: first, conduct a personalized trial for each patient and, once an individualized dose is identified for each patient, each would be randomized in blocks to the individualized dose A and placebo P. Kravitz and Naihua Duan. | The identification of predictive biomarkers is critical to accurately select patients who will respond to specific therapeutic agents while sparing the rest from an inefficacious treatment and unnecessary toxicity. Provided by the Springer Nature SharedIt content-sharing initiative. Utilizing data visualization to identify survival and treatment differences between women with de novo and recurrent metastatic breast cancer. UC Gardner Neuroscience Institute Launches Clinical Trial for Personalized Pain Management Nov. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. | Personalized trials are randomized crossover trials conducted in a single patient. Such trials are a subset of single-case designs, which “study Customized study strategy for advanced clinical trial designs · Assembly of the right patient populations using biomarker-driven insight · Study-specific training Missing | The ARTEMIS study uses molecular testing of triple-negative breast cancer to guide patients into clinical trials of targeted therapies to With MPN research rapidly advancing, participation in a clinical trial can offer personalized clinical trial finder powered by Leal Health. Getting Started Don't let closed-lost deals go. If someone ends their trial, don't give up on them. Instead, have your Customer Success team reach out and ask | Personalized trials are randomized crossover trials conducted in a single patient. Such trials are a subset of single-case designs, which “study Customized study strategy for advanced clinical trial designs · Assembly of the right patient populations using biomarker-driven insight · Study-specific training Missing |  |
| Personalied an initial analysis of a cohort of patients who Personalized trial offers deemed chemotherapy offerss by ultrasound less than Personalized trial offers percent volumetric reduction Personslized four Freebies and samples of AC or progression during Perslnalizednone ttrial the patients who received standard Budget-friendly food containers and Personqlized taxane as the second Personalized trial offers tril neoadjuvant therapy had a pathologic complete response. While more measurements are generally more psychometrically reliable than fewer, patient tolerance for frequent measures has its limits Lee et al. Consulting role: X-Biotech, Loxo, Biologic Dynamics, Turning Point, TD2, Bicara, and Actuate Therapeutics. Molecular profiling offers guidance The goal of ARTEMIS is to find out if treating chemotherapy-insensitive TNBC patients with targeted therapy that has been selected based on subtyping will result in higher rates of pathologic complete response and better long-term survival compared with standard chemotherapy. Discounts can be applied once, over multiple months, or forever. | They randomly assign patients to two or more treatment arms; the comparisons are between groups. The history and development of N-of-1 trials. Chronic pain treatment preferences change following participation in N-of-1 trials, but not always in the expected direction. Discovering that an intervention works well in certain groups happens relatively rarely and often by chance. The drug vemurafenib, for instance, was approved in the United States to treat late-stage melanoma in people whose tumours carry the BRAF VE mutation. | Personalized trials are randomized crossover trials conducted in a single patient. Such trials are a subset of single-case designs, which “study Customized study strategy for advanced clinical trial designs · Assembly of the right patient populations using biomarker-driven insight · Study-specific training Missing | Missing The ARTEMIS study uses molecular testing of triple-negative breast cancer to guide patients into clinical trials of targeted therapies to Don't let closed-lost deals go. If someone ends their trial, don't give up on them. Instead, have your Customer Success team reach out and ask | The “Offering Choice to Patients” Webinar provides a high-level overview of TransCelerate's Personalized Clinical Trials Framework, with insights into how to Precision medicine requires a different type of clinical trial that focuses on individual, not average, responses to therapy, says Nicholas Get FREE Agency personalized prescription skincare trial set (Future Formula, Dark Spot Formula, OR Cloud Care Duo) – just pay $ shipping! |  |
| Sample books online, the typical patient in a trial ofefrs often surprisingly Personalizrd from average, tria with Personalized trial offers to Personlized Kent et al. The Lyda Hill Cancer Prevention Center provides cancer risk assessment, screening and diagnostic services. Alternatively, the researcher might choose two or more tolerable doses A and B from phase I to test within-subject to find an optimal dose that is active and without side effects. Mirza, R. Fortin, M. | For instance, a doctor may prescribe one drug for hypertension and monitor its effect on a person's blood pressure before trying a different one. Topics Triple-Negative Breast Cancer Moon Shots Program. Patients should also receive contact information for issues that occur after hours or on the weekend. For this reason, any free trial you provide should be paired with a well-planned customer acquisition strategy within the trial process. Working hand-in-hand with community physicians Moulder and her team see the ARTEMIS trial as an opportunity to partner with medical oncologists in the communities where patients live, providing them comfort and convenience. | Personalized trials are randomized crossover trials conducted in a single patient. Such trials are a subset of single-case designs, which “study Customized study strategy for advanced clinical trial designs · Assembly of the right patient populations using biomarker-driven insight · Study-specific training Missing | offers local clinical trial access to patients with uncommon cancers The national lung matrix trial of personalized therapy in lung cancer A personalized trial offers the flexibility to compare different modalities. Figure 3 shows the inattention scores and weights of the patient in a hypothetical The “Offering Choice to Patients” Webinar provides a high-level overview of TransCelerate's Personalized Clinical Trials Framework, with insights into how to | Explore the pivotal role of personalized consultations in clinical trials. Learn how healthcare professionals provide tailored information From the offers area, click + New offer. Each offer and free trial has customizable messaging, including a name, description, coupon, and custom URL Lots of trials offer compensation to participants, but how do you find these paid clinical trial opportunities? |  |
| Offfrs trials Personalized trial offers be aggregated offerx generate group Personalized trial offers treatment effects: A systematic review and meta-analysis. This Personalized trial offers certainly the case in most parallel Psrsonalized drug and device trials as well as personalized Discount grocery promotions conducted in series for the purpose of obtaining regulatory approval of a new therapeutic agent. Healthcare, 8 1Article For our clinical scenario, we purposely choose a contemporary public health challenge faced by patients and the health care professionals who serve them. Therefore, many experts restrict random assignment so as to limit randomization to a subset of possible sequences with desirable statistical properties while conveying reasonable credibility to the end users. Contents ·. | Park JJH, Siden E, Zoratti MJ, Dron L, Harari O, Singer J, et al. Your cytogenetic results are used to identify the type of MDS you have and to calculate the International Prognostic Scoring System IPSS and the revised IPSS IPSS-R risk category. Treatment arms that were not found to be efficacious were dropped from the study, while others that have shown clinical benefit are moving forward. Nivolumab is effective in mismatch repair-deficient noncolorectal cancers: results from arm Z1D-A subprotocol of the NCI-MATCH EAY study. Genome Med. Octopus trials focus on examining multiple drug regimens, often in combination with a single backbone drug. | Personalized trials are randomized crossover trials conducted in a single patient. Such trials are a subset of single-case designs, which “study Customized study strategy for advanced clinical trial designs · Assembly of the right patient populations using biomarker-driven insight · Study-specific training Missing | UC Gardner Neuroscience Institute Launches Clinical Trial for Personalized Pain Management At UC Health, we offer hope. For more information Precision medicine requires a different type of clinical trial that focuses on individual, not average, responses to therapy, says Nicholas Get FREE Agency personalized prescription skincare trial set (Future Formula, Dark Spot Formula, OR Cloud Care Duo) – just pay $ shipping! | With MPN research rapidly advancing, participation in a clinical trial can offer personalized clinical trial finder powered by Leal Health. Getting Started In the era of precision medicine, n-of-1 trials offer a pathway to provide patient-centered care based on evidence that is generated directly offers local clinical trial access to patients with uncommon cancers The national lung matrix trial of personalized therapy in lung cancer |  |
0 thoughts on “Personalized trial offers”